15+ Calculate The Mole Fraction Of Glycerol In This Solution
Calculate the mole fraction of glycerol in this solution. As the volume of the solution made was 1000L moles of glycerol are.

Slow Magnetic Relaxation In A Co Iii Co Ii Co Iii Mixed Valence Complex With Negative Anisotropy Crystal Growth Design
Web Calculate the mole fraction of the solute and solvent.
. How do you calculate mole fractions. In the given mixture sum of all the mole fractions is equal to one. Calculate the concentration of the glycerol solution in percent by mass.
Of moles of CH 3 OH no. X A X B 1. Express the mole fraction to four significant figures.
Add your answer and earn points. 25 by weight means 25 g is dissolved in 100 gram of solution. Advertisement johnmo799 is waiting for your help.
Web Calculate the mole fraction of aqueous glycerol C₃H₈O₃ solution in 25 by weight of glycerol. Web The solution is prepared by mixing 250 grams of ethanol and 250 grams of water. Determine the mole fractions of each component.
Web Calculate the mole fraction of glycerol in this solution. Now here in discussion we have not given that what the solvent is. The multiplication of the mole fraction by 100 gives the mole percentage.
Web Calculate the mole fraction of glycerol in this solution. Enter the moles of solute solvent and x for the unknown in the respective input field Step 2. The mole fraction of glycerol is 00613.
Web The procedure to use the mole fraction calculator is as follows. Calculate the concentration of the glycerol solution in parts per million. Web You can read how to calculate mole fractions in.
In your problem n_glycerol 92 g glycerol 1mol. X CH3OH 10 10. Of moles of CH 3 OH no.
Mole fraction of water is 071. Calculate the concentration of the glycerol solution in percent by mass. Web We calculate the modularity of a visceral solution in the past part of the related in the first part of the question but which is equal to the modularity of a restaurant solution The.
Calculate the concentration This problem has. So in such a case we. Web Please explain Summate the mole fraction and molality of glycerin in a solution prepared by dissolving 450 grand of glycerin C_3H_5OH_3 in 100 g H_2O.
Of moles of H 2 O. X CH3OH no. Web A solution of 2950x10²M contains 2950x10² moles of Glycerol per Liter of solution.

Pdf Conductivity Surface Tension And Comparative Antibacterial Efficacy Study Of Different Brands Of Soaps Of Nepal
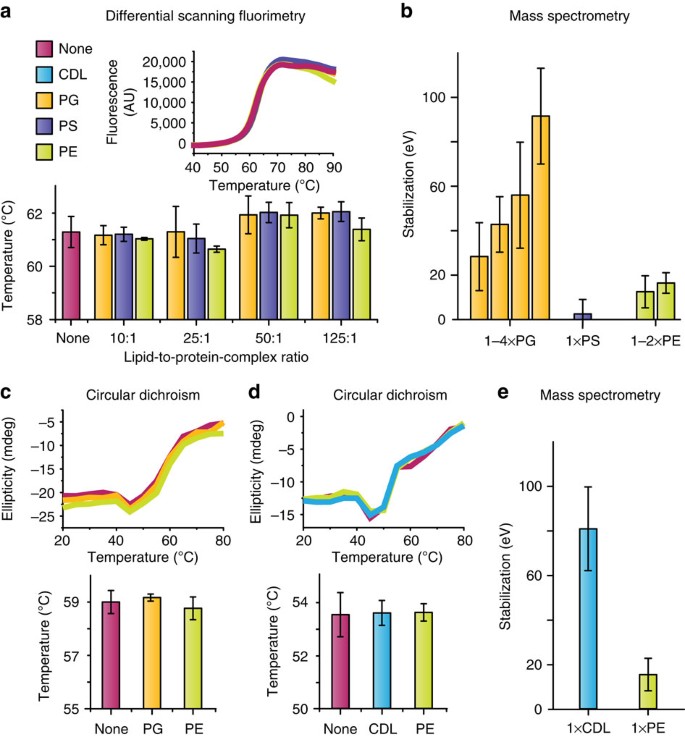
Quantifying The Stabilizing Effects Of Protein Ligand Interactions In The Gas Phase Nature Communications

Step 1 2 Flashcards Quizlet

What Is The Mole Fraction Of 10 Urea Solution Brainly In

Pdf Concise Biochemistry Mcqs
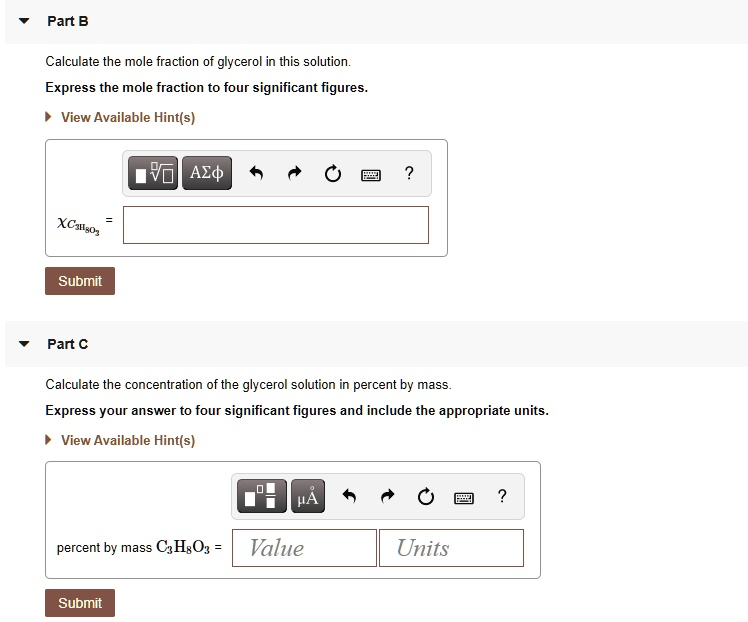
Solved Pant B Calculate The Mole Fraction Of Glycerol In This Solution Express The Mole Fraction To Four Significant Figures View Available Hintfs Azd B Submit Pant C Calculate The Concentration Of

Calculate The Mole Fraction Of Glycerol Solution In 25 By Weight Of Glycerol Brainly In

Solved Correctly Order The Steps Required To Calculate The Chegg Com

Mole Fraction Of Glycerine In A Solution Is 36 Gram Of Water And 46 Gram Of Glycerine Is Brainly In
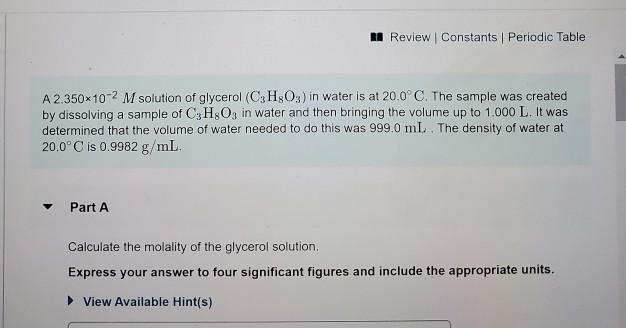
Solved 5 Of 10 A Review Constants Periodic Table A Chegg Com

Welcome To Chem Zipper Com The Mole Fraction Of Glucose In Aqueous Solution Is 0 2 The Molality Of Solution Will Be
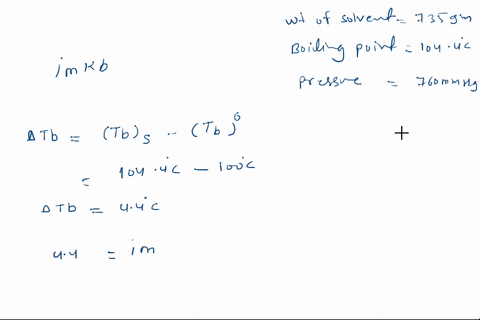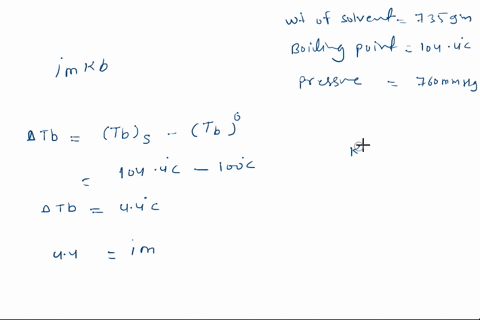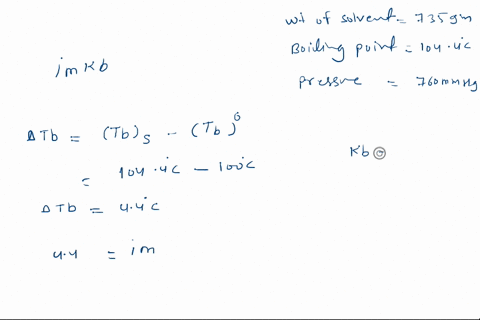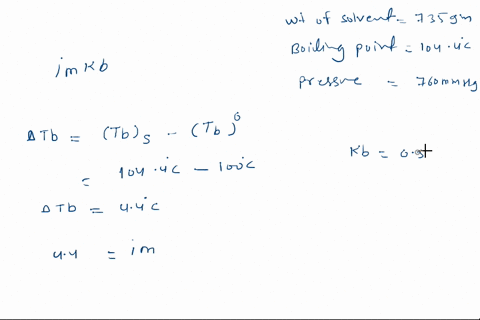
Solved Calculate The Mole Fraction Of Glycerol Solution In 25 By Weight Of Glycerol

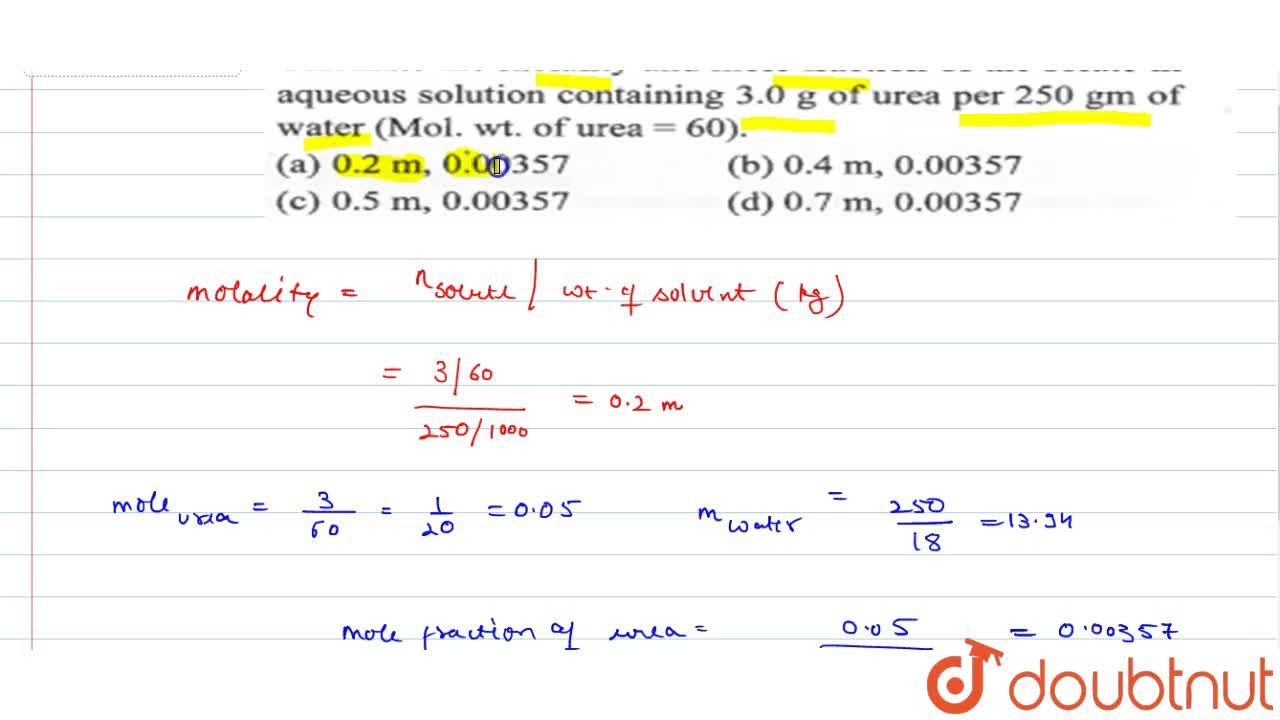
Calculate The Molarity And Mole Fraction Of The Solute In Aqueous Solution Containing 3 0 G Of Urea Per 250gm Of Water Mol Wt Of Urea 60

Calculate The Mole Fractions Of The Components Of The Solution Composed By 92g Glycerol And 90g Water M Water 18 M Glycerol 92

Solved Pant B Calculate The Mole Fraction Of Glycerol In This Solution Express The Mole Fraction To Four Significant Figures View Available Hintfs Azd B Submit Pant C Calculate The Concentration Of

Chasing The Co Crystal Disappearing Polymorph With Ab Initio Methods Crystal Growth Design
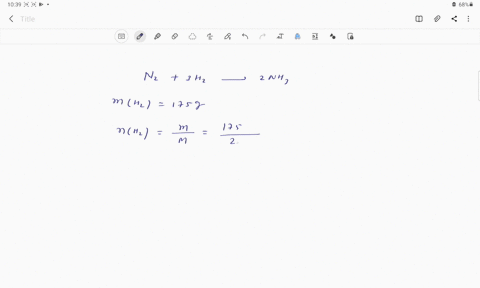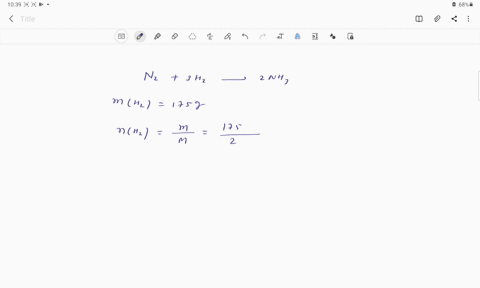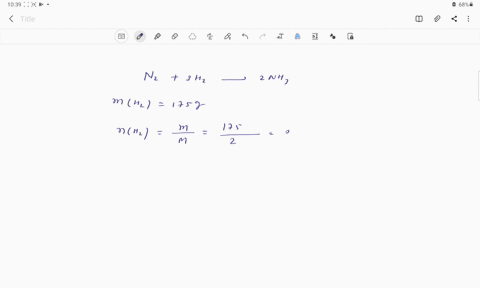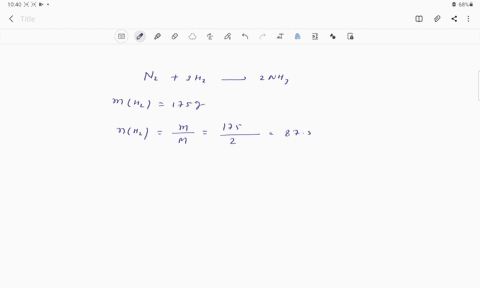
Solved Pant B Calculate The Mole Fraction Of Glycerol In This Solution Express The Mole Fraction To Four Significant Figures View Available Hintfs Azd B Submit Pant C Calculate The Concentration Of